The creators of a device that delivers small electrical waves to the brain are calling this technology a revolutionary treatment for mental health. However, some individuals remain skeptical about its effectiveness.

According to Saadnews' Science and Technology Service, citing Faradeed, the Swedish company Flow claims it can help most people recover from depression. Over the past year, the company has made headlines by introducing a device for “innovative brain stimulation” that patients can use at home.
Flow users receive a headset that sends small electrical pulses to a part of the brain called the "dorsolateral prefrontal cortex." This area is associated with decision-making, motivation, planning, and working memory—functions that are often disrupted by depression. According to Alex O'Neill, a psychiatrist specializing in neuromodulation for depression, the idea stems from research that shows depression is linked to weak neural connectivity on one side of the brain. O'Neill refers to brain imaging studies using positron emission tomography (PET) that show the glucose usage patterns in the brains of depressed individuals differ from those of non-depressed people.

O'Neill explains, "If you look at the brain of a non-depressed person, glucose use is balanced on both sides. But in the brain of a depressed person, the left side seems inactive, while the right side is often overactive. So, the idea emerged that depression may be related to poor connectivity on the left side of the brain, and perhaps we can find a solution."
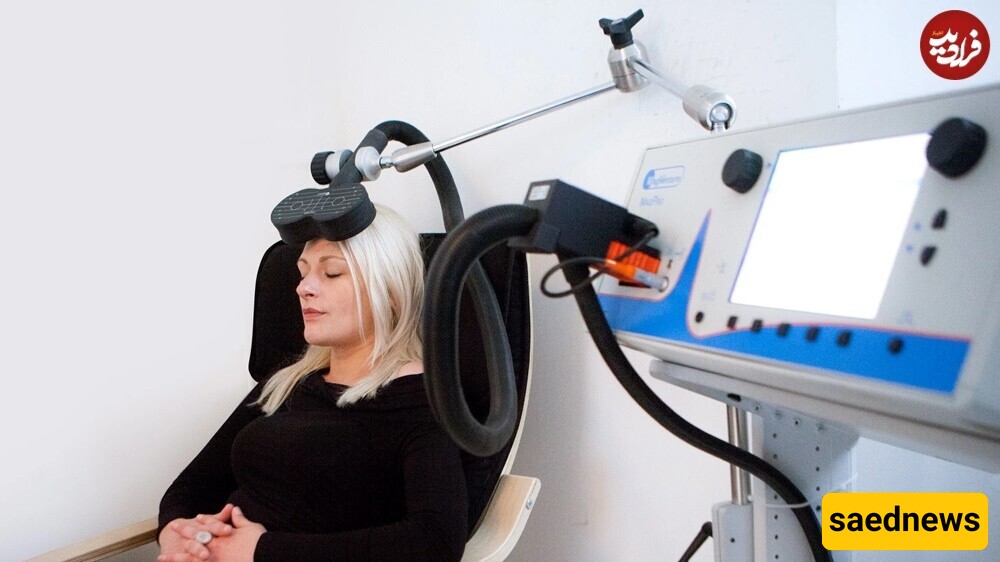
This led to the development of repetitive transcranial magnetic stimulation (rTMS), which uses magnetic fields to stimulate the left side of the brain while suppressing the right side. In the United States, rTMS devices and protocols have been approved for treating depression, and clinical trials with patients ranging from mild to severe depression have shown positive results.
While rTMS is now widely used in various countries, its use in the UK remains limited due to the high costs of purchasing devices and setting up specialized treatment centers.
Flow technology is based on a similar method called transcranial direct current stimulation (tDCS), which aims to achieve the same effects as rTMS by using low-power electrical currents instead of magnetic fields. Since this approach is safer, patients can use it at home, making it much more affordable.
In October 2024, a Phase 2 clinical trial with 174 depressed patients yielded promising results. The published data revealed that 45% of patients who received active treatment with the Flow device showed improvement over 10 weeks, while only 22% of those who received the inactive (placebo) version of the device showed improvement.
Nord and other independent mental health researchers note that key questions remain unanswered.
Across social media and online forums, many individuals have shared reports claiming that the treatment helped improve their symptoms. Frank Padberg, a professor of psychiatry and psychotherapy at LMU University Hospital in Munich, says these personal reports should not be dismissed. However, he acknowledges that evaluating a technology based solely on individual stories is challenging.
Padberg and Nord explain that while Flow's clinical trial showed positive results, other independent tDCS trials for depression have shown limited effects. When Padberg led a six-week tDCS treatment trial in 2023, the results did not show any significant reduction in depressive symptoms between the active and inactive tDCS devices. While tDCS may be a helpful complementary treatment for psychotherapy or medication, it is currently best to expand its use experimentally so researchers can determine which patients benefit the most from this treatment.

