Electric charge is a fundamental property of every material, carried by certain fundamental particles, and it controls how particles interact under the influence of an electric or magnetic field. Electric charge, which can be positive or negative, occurs in discrete natural units and is neither created nor destroyed.

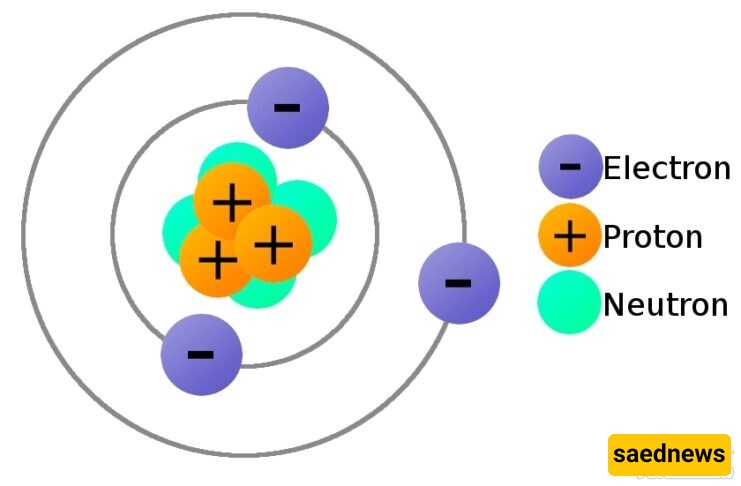
Electric charge (in English: Electric charge) (abbreviated as charge) is a property of matter (materials around us) that causes a force to be exerted on it when a charged object is placed near another charged object. Electric charge comes in two types: positive and negative. When two materials or objects have the same type of charge, they exert a repulsive force on each other, while if they have opposite charges, they attract each other. In the International System of Units (SI), the unit of electric charge is the coulomb (C). However, in electrical engineering, the ampere-hour (Ah) unit is also used. In interactions between charged bodies, classical electromagnetism is sufficient, and quantum effects are neglected. Electric charge is a conserved property of matter, referred to as the law of charge conservation, which states "the algebraic sum of all electric charges in an isolated system remains constant." In other words, this means that it is impossible to create or destroy a net charge; charge is transferred from one body to another. Electric charge originates from subatomic particles of matter that determine the electromagnetic properties of the material. A charged material generates electromagnetic fields and is also influenced by them. The interaction between a moving charge and an electromagnetic field generates electromagnetic forces, one of the four fundamental forces. Experiments in the 20th century have provided a quantum explanation of electric charge (a process known as quantization), and scientists have discovered that electric charge is composed of a smaller unit called the elementary charge. The charge of an electron is approximately {\displaystyle e=1.602\times 10^{-19}C}. (There are also particles called quarks that have charges of about a third of the elementary charge, e⅓.) A proton has a charge equal to e, and an electron has a charge of e-. The study of charged particles and their interaction with photons is called quantum electrodynamics. The charge of an object is the number of free electrons or protons it contains. The definition of the size of charge: The amount of electricity in an electron or proton is {\displaystyle 1.6\times 10^{-19}C}. If this is multiplied by the number of electrons or protons, the result is the charge of the object.
History of Electric Charge: Thales, the 6th-century BC Greek philosopher, said that rubbing a piece of fur on various materials such as amber could produce a charge or electricity. The Greeks also stated that charged amber buttons could attract lightweight objects like hair, or rubbing amber for a long time might produce sparks. In 1600, the English scientist William Gilbert revived the discussion of electricity and coined the Latin word "electricus," derived from the Greek word "ήλεκτρον," meaning amber. This term soon transformed into the English terms "electric" and "electricity." In 1660, Otto von Guericke continued Gilbert’s work and is likely the inventor of the device that produces static electricity. Other European pioneers in the field include Robert Boyle, who in 1667 stated that electric attraction and repulsion could occur in a vacuum. In 1729, Stephen Gray classified materials into conductors and insulators. Charles François de Fé in 1733 stated that electricity comes in two different types that can neutralize each other. He proposed the theory of "two fluids," stating that when glass is rubbed with silk, it becomes charged or "glass charge," while when amber is rubbed with fur, it becomes charged with "resinous charge." In 1839, Michael Faraday showed that the apparent division between static electricity, current electricity, and bioelectricity was incorrect and all of these arise from the behavior of different poles of dipoles, which we arbitrarily call positive and negative. Positive charge is the charge remaining on a glass rod after rubbing it with silk. Benjamin Franklin, in the 18th century, was the most experienced in this field. He supported the theory of a single electric fluid. He believed that electric charge was an invisible fluid present in all materials. For instance, he believed that glass stored electrical charge in a Leyden jar. He demonstrated that rubbing two insulator surfaces together caused this fluid to shift, and flowing of this fluid created electric current. He also proved that if a material had a small amount of this fluid, it had a negative charge, and if it had an excess, it had a positive charge. Arbitrarily (or for reasons not recorded), he chose the charge on the glass rod to be positive and the resinous charge to be negative. He also introduced the terms "charge" and "battery" into electrical vocabulary. William Watson also reached similar conclusions around the same time as Franklin.
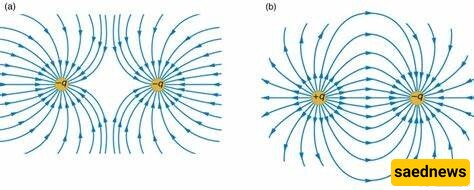
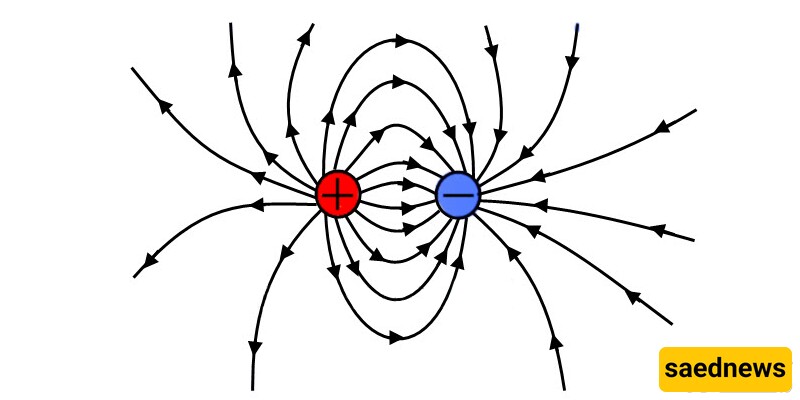
Characteristics of Electric Charge: Electric charges are of two main types: positive and negative. Two objects that have the same type of charge, when relatively close, exert a repulsive force on each other. When they have opposite charges, one positive and the other negative, they attract each other. In addition to the existence of two types of charges, several other features of charge have been discovered.
Static Electricity and Current Electricity: Static electricity and current electricity are two separate phenomena caused by electric charge, and both can occur simultaneously in a body. Static electricity is a source of electric charge in an object, and if two objects that are not in electric equilibrium are brought into contact, an electrical discharge occurs between them. Electrical discharge alters the electric charge of both objects. On the other hand, current electricity is the flow of electric charge in a body, which does not result in the loss or gain of charge in that body. Although in electrical discharge, charges flow from one object to another, the flow is too brief to be considered electric current.
Properties of Electric Charge: In addition to all the electromagnetic properties of electric charge mentioned above, charge is a relativistic variable, meaning that any particle with charge Q, no matter how fast it moves, is assumed to always maintain its charge Q. This property of charge has been demonstrated by experiments, for example: the charge of a helium nucleus (with two protons and two neutrons moving at high speed) is equal to the charge of two deuterium nuclei (with one proton and one neutron moving at a much slower speed).
Methods of Transferring Electric Charge: First, we need to understand the concept of conductors and insulators:
Conductors: Materials that have free electrons.
Insulators: Materials that lack free electrons.
Methods of Charge Transfer:
Friction: This is typically done with insulators. When two objects are rubbed together, they become electrically charged. One gains electrons (negative charge), and the other loses electrons (positive charge).
Contact: This is usually done with conductors. When two conductive objects come into contact, they transfer charges to each other relative to their volumes. In conductors, electrons move quickly and distribute evenly. In insulators, electrons move slowly and remain at the contact point.
Induction: This method is also used with conductors. By using one or two insulating spheres, we can induce charge. For example, bringing a negatively charged rod near a sphere will cause the negative charges to move away. If the sphere is connected to the ground using a conducting wire, negative charges will flow out, and after disconnecting the wire and removing the rod, the sphere will remain charged.

